Fingerprinting - Equivalence
Fingerprinting and equivalence testing of materials is at the core of quality assurance (QA) and quality control (QC). The primary goal of QA/QC is to demonstrate that a product be it chemical, pharmaceutical, or biomedical device is the same, i.e. “equivalent”, from batch to batch. Mass spectrometry provides not only the most sensitive but also the most specific analytical option for demonstrating chemical equivalence.
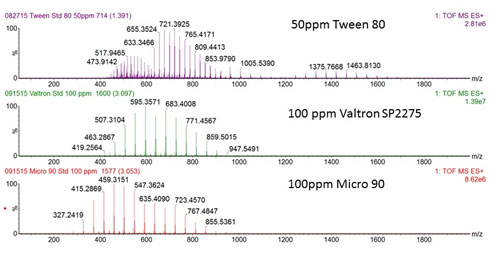
Mass spectral “fingerprints” provide highly resolved chemical images of a sample, much more specific than FTIR which is a chemical fingerprinting analytical tool routinely employed in QC/QA testing. The mass spectra of three different aqueous non-ionic detergent solutions in the figure below display obvious differences even though the three surfactants are chemically very similar.
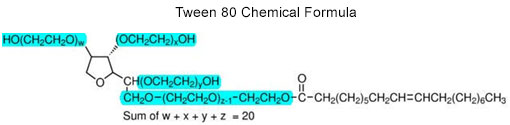
These non-ionic surfactants are mainly comprised of polyoxyethylene (POE) hydrophilic polymer units and thus one might expect their mass spectra to be equivalent. In fact, they are not equivalent due to fine differences in chemical structure that high resolution accurate mass spectrometry can readily detect. POE polymer chains differ in their molecular weight, i.e. number of repeats, and are also terminated or otherwise functionalized by distinguishing chemical groups as illustrated in the idealized structure of Tween 80 (PEO moiety is highlighted in blue in the lower figure; the end group is unhighlighted). These differences in molecular weight and functional group character are the structural features that lead to the conspicuously different surfactant fingerprints.
This surfactant example provides only a glimpse of the power of mass spectral fingerprinting. Similar data can be used in a multitude of QA/QC applications. For example, assurance of lot‑to‑lot chemical equivalence at incoming receiving with a "gold standard" fingerprint is a worthy application especially for critical and sensitive materials such as biomedical device adhesives.
As another example, alternate component sourcing can also be expedited by mass spectral fingerprinting. All experienced engineers, at one time or another, have been faced with an unexpected material source change with limited time and money to qualify a new source and implement the new material into the process. By showing indisputable chemical similarity/equivalence, mass spectral fingerprinting can fast track this process.